Scientists confirm original tetrahedral model of molecular structure of water
Scientists confirm original tetrahedral model of molecular structure of water
Whew! I'm glad they finally put that debate on ice...pun intended.
Whew! I'm glad they finally put that debate on ice...pun intended.

What was interesting to me about this article was that there was a debate at all. I do not know much about the history of chemistry or when/how compounds were discovered. I would not have guessed that the structure of water would be a debated topic. Water is everywhere and a very important aspect of life. It is interesting that there are still experiments to discover a deeper understanding of its structure. In fact, I was researching about this topic and found out that others are baffled about this debate as well. One article stated, “ ‘If we don't understand this basic life material, how can we study the more complex life materials—like proteins—that are immersed in water?" asked Postdoctoral Researcher Congcong Huang, who conducted the X-ray scattering experiments. "We must understand the simple before we can understand the complex.’” 1 I agree with this; now that the structure of water has been changed back to its original understanding, would other ideas that were based off this new structure have to change? It seems that this new structure makes better sense being that hydrogen bonding in water is what makes the molecule so interestingly different from others. There’s another point that confused me about this article. The article says that the bonding between only two molecules was probably seen during an “instantaneous asymmetrical fluctuation”. This does not makes sense to me; if the fluctuation is rare and only occurs for a very brief amount of time, then how did the scientist happen to catch it at that moment? Were there not multiple tests done at different time periods? If so, what are the odds that this rare fluctuation occurred multiple times? This seems very fallible and the quadruple bond theory seems more assuring.
ReplyDelete1. http://www.sciencecodex.com/scientists_working_to_unravel_the_molecular_structure_of_water
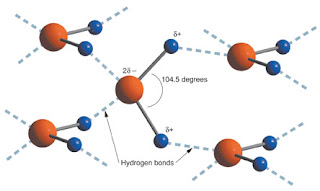
Water is arguably the most important biological compound, composing about 70% of the planet and 60% of the human body. Its structure gives rise to numerous properties that have significance almost everywhere in nature, particularly in chemistry. Its unique structure is what allows for much of its distinguishing functions, such as hydrogen bonding and water's unusually high heat capacity. It has been thought, for over 100 years, that water's structure is tetrahedral. This bent tetrahedral structure is responsible for water's high polarity and ability to form bonds with four other molecules. It's because of this structure and its polarity that water is extremely useful as a solvent, capable of serving as both a base and an acid. Apparently, this traditional view of the structure of water is disputed. I too find this odd, as the tetrahedral structure of water is widely accepted. In fact, this structure of water is all I have been taught in my scientific career! It is odd to think of this being disputed. I do like how the scientists who have confirmed the tetrahedral structure addressed the arguments of the people who thought they had disproved it in 2004. I like how they used the electron modeling theory to describe that what the other researchers saw was just a "snapshot" of the real tetrahedral interaction of water. This is because the true electron structure of water is not stagnant and constantly moving, which causes the temporary asymmetrical structure of water, while still retaining the overall tetrahedral structure. All in all, I found this article to be interesting, even if it does seem less important at this point in the evolution of science (pun).
ReplyDeleteI think it is amazing that it was a debate worthy topic. I always thought that since water was such a common molecule, everything was known about it, especially something as basic as the structure. However, I will admit that I never really thought about the 3D structure of water, I always picture it as a linear, bent molecule. This article was really eye opening to me when it came to the structure. Learning that water can bond to four places seem obvious, but something that tends to get overlooked when just talking about the three molecules attached to it. Strong evidence from Berkeley lab push towards the continuum model, which is shown by Richart Saykally, stating, “Many of the features of… the two state model actually result from a continuous distribution of intact hydrogen bonds. (1) This proves the tetrahedral model, because there is four bonding sites. Also, another thing I found interesting was that the image shown of the water molecule looks like a smiley face.
ReplyDelete1. http://www.lbl.gov/Science-Articles/Archive/sabl/2005/October/03-water-contoversy.html
The tetrahedral shape of water is extremely to the survival of all organisms on earth. The tetrahedral shape of water is due to the two pairs of lone electrons from the oxygen and the two hydrogens bonded to the oxygen. The two pairs of lone electrons strain the hydrogen to oxygen bonds causing the molecule to have a bent shape. This structure gives water molecules a very strong dipole. Water molecules are also capable of hydrogen bonding. The strong dipole along with hydrogen bonding allows water to be a very dense liquid. It also gives water it’s relatively high boiling point. Both these properties are a result of the extremely organized intermolecular structure of water. The high density of water in its liquid state allows ice to floats on top of a body of water instead of sink. Organisms that live in lakes or other body of water that freeze over would not be able to survive if ice was denser than liquid water. Entire bodies of water would turn to ice instead of only having a top ice layer. The high boiling point of water is what allows life on earth to be possible. If water boiled at room temperature we would have no effective way of storing water inside our bodies. The strong dipole of water also allows it to interact with other polar molecules. This is vital to many metabolic pathways within our bodies.
ReplyDeletehttp://witcombe.sbc.edu/water/chemistrystructure.html
http://www.sciencedaily.com/releases/2009/08/090811143716.htm